News
Professor Chiara Bonini: the engineering of T cells
We are happy to announce that Chiara Bonini, Group Leader of the Experimental Hematology lab at IRCCS San Raffaele Hospital and Full Professor in Hematology at Vita-Salute San Raffaele University, has been honored by the American Society of Hematology (ASH) to give this year’s Ham-Wasserman lecture.
The Ham-Wasserman lecture is one of the plenary sessions scheduled for the annual ASH meeting, the most important world congress of Hematology, which every year gathers health care providers, scientists, physicians and biotech representatives working in the field.
The lecture, whose name derives from the names of the two eminent hematologists Thomas Hale Ham and Louis Robert Wasserman, is assigned every year to the non-US scientist who has distinguished him or herself for his/her studies in Hematology.
Professor Chiara Bonini pioneered the clinical use of genetically engineered lymphocytes in stem cell transplantation, leading to the first cell-based gene therapy product approved by EMA for tumors. More recently, her team developed the T Cell Receptor (TCR) gene editing approach to completely and permanently redirect T cell specificity against cancer.


Professor Chiara Bonini
Professor Chiara Bonini, what does the Ham-Wasserman lecture mean to you?
Aside from being one of the most important recognitions for hematologists, for me the Ham-Wasserman lecture has a special meaning, since I started my career in research at the annual ASH conference, just after graduation from the Medical School.
Back then, I had joined the lab of Professor Claudio Bordignon, a pioneer of gene therapy and the founder of the San Raffaele-Telethon Institute for Gene Therapy (SR-Tiget), who assigned me a thesis project on the use of suicide genes in cancer immunotherapy. It was a preclinical work that eventually led to the start of the first clinical trial that used engineered T cells to treat hematological cancers relapsing after allogeneic hematopoietic stem cell transplantation.
On the year of my graduation, 1994, the results of the project were chosen to be presented in the prestigious Plenary Session of the ASH congress and Professor Bordignon trusted that I could present the project in front of thousands of scientists.
Fast forward, this year I am going to present the state-of-the-art of my work in T cell engineering at the annual ASH congress, as the speaker of the Ham-Wasserman lecture. Career wise, thus, this acknowledgment is truly important to me, it’s kind of a circle that closes...
What was the topic of your master thesis?
Back then, we had understood that when doing bone marrow transplantations in cases of acute myeloid leukemia we were transferring not only the stem cells needed to regenerate healthy hematopoiesis in the receiving patient, but also T lymphocytes that were kind of “contaminating” the transplant. Importantly, we observed that these donor T cells could kill tumor cells in the host, leading to what we call the graft versus leukemia effect.
However, such unexpected immunotherapeutic effect of bone marrow transplantation came with a cost: donor T cells could kill leukemic cells but, if unrestrained, they could also kill the host’s own cells, eventually leading to the graft versus host disease– that is, the transplant acting against the host. So, my thesis project aimed at keeping the graft versus leukemia effect while preventing the graft versus host disease.
To restrain T cells and keep only their anti-tumor action, we had introduced a suicide gene in T cells. If the graft versus host disease ensued after bone marrow transplantation, suicide genes could be activated in T lymphocytes upon administration of a specific drug, thus leading to T cell death and control of unwanted side effects.
This work paved the way for the development of current therapies based on T cell engineering, including CAR-T and TCR cell therapies, which leverage the immunotherapeutic action of T cells to treat several diseases.


IRCCS San Raffaele Hospital
Tell us a bit more about CAR-T and TCR cell therapies.
The idea at the basis of both therapies is to instruct T lymphocytes to kill “unwanted” cells, like cancer cells. To do this, you need T cells to recognize those cells, and this can be achieved either by engineering them with a Chimeric Antigen Receptor (CAR) or with an anti-tumor T Cell Receptor (TCR).
CARs are synthesized in the lab, and they combine the advantages of humoral immunity, because the antigen recognition occurs via a structure resembling the variable portion of antibodies, with the cytotoxic action of T lymphocytes in which such receptors become expressed. CARs usually recognize surface antigens which are presented in their native conformation.
However, most tumor-specific antigens are not on the surface of cancer cells, but instead inside the cells, and cannot be targeted by currently available CARs. For example, nowadays we have some approved CAR-T cell therapies to treat hematological malignancies like acute lymphoblastic leukemia (ALL) and several non-Hodgkin lymphomas. These CAR-T cells can detect CD19, which is a surface antigen expressed by both healthy B cells and cancer cells. This means that T cells harboring CARs that detect CD19 will kill both unwanted leukemic cells as well as normal B cells. Since we can compensate for the lack of B cells, we can pay the price to get rid of them while having effective therapy against severe cancers like ALL.
However, when it comes to other types of cancer, it is indispensable that CARs recognize more specifically tumor antigens. This is the focus of current research in CAR-T cell therapy: screening tumors (especially solid neoplasms, for which CAR-T cell-based therapy is still challenging) for cancer specific antigens that can be selectively detected by CARs. Recently the team of Monica Casucci, at our Institute has developed a new CAR specific for colorectal cancer and we are eager to further develop this innovative engineered lymphocyte.
On the other hand, here is where TCR based T cell therapies could come in handy. TCRs are naturally present on T cells. Some of these naturally occurring TCRs can detect only cancer-specific antigens. Our task, thus, is to look for T cells expressing those anti-tumor TCRs, expand them and extract the genetic information needed to clone those anti-tumor TCRs to engineer new T lymphocytes only against cancer.
The tricky part, here, is to screen for these rare, but naturally occurring, anti-tumor TCRs and select them to re-direct T lymphocytes against as many kinds of tumor as possible. This is what we are currently doing thanks to the work of Eliana Ruggiero: she has developed a pipeline to identify new TCRs that can redirect T cells against more than 60 different tumor antigens.
In the selection of the most proper CAR and TCRs to be implemented in clinical testing we are all guided by the team of Luca Vago, which is involved in the identification of the mechanisms by which cancer cells, and leukemic cells in particular, avoid the immune response. Thus, at our Institute we have an excellent scientific community devoted to the development of CAR and TCR-based based therapy.
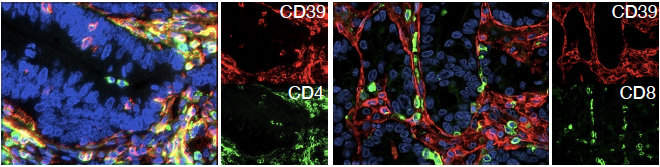

Liver metastasis of colorectal cancer showing T lymphocytes (green) expressing CD39 (red), a novel target for gene therapy with engineered lymphocytes
What are the milestones that significantly marked your career in research?
I think that my career was marked more by the people I met, rather than my scientific achievements.
First, I’m talking about Claudio Bordignon: he was a great mentor: he was an example of passion, vision and ambition, he challenged me with difficult scientific questions and trusted me, to the point of sending me, just after graduation, to present our project in the plenary session of the annual ASH conference. That was a brave and generous choice, that started my scientific career, and allowed me to enter other excellent Institutes, and to learn from incredible mentors, such as Richard O’Reilly in MSKCC, a pioneer of hematopoietic stem cell transplantation.
Then, I’m thinking of Fabio Ciceri, a knowledgeable and eclectic physician scientist, who combines the urgency of unmet clinical needs, with extensive expertise in clinical trial design and the ability to implement innovative therapeutic approaches. These are essential characteristics to properly tackle fundamental questions in Medicine and develop new therapies. Of course, my postdoc at the Fred Hutch in Seattle, in the lab of Phil Greenberg, a founder of all T cell-based medicine, was crucial for me to learn more about immunology and T lymphocytes and all of this helped me to lay the foundations of my lab when I was back in Milan.
Around 2008-2010, my main research question revolved around how to insert selected anti-tumor TCRs in T cells while getting rid of their native TCRs. In other terms, I needed the genetic tools to remove the genes encoding native TCRs from T cells and insert the genes encoding TCRs that can recognize tumor antigens. Nowadays this is easily achieved with CRISPR/Cas9, but back then such a task of replacing genes was very laborious.
Here is where I had the privilege to start a collaboration with Luigi Naldini, inventor of lentiviral vectors and pioneer of gene transfer and genome editing technologies. At the time, his lab was testing zinc finger nucleases as genome editing tools. Through this collaboration, which also included Phil Greenberg, we developed the TCR gene editing concept. It took us quite an effort and a while, but eventually we managed to completely redirect the specificity of T lymphocytes, thus enforcing recognition and killing of cancer cells.
This work, which was published in Nature Medicine in 2012, laid the foundations for the techniques we are still using in several labs in the world, to engineer T cells for immunotherapy.
Today, thanks to the availability of user-friendly genome editing tools, such as CRISPR/Cas9 and base editors, we can efficiently edit the T cell specificity while also manipulating the T cell genome, to build Super-T cells able to recognize cancer cells and resist the impervious and hostile microenviroment of tumors. Based on this concept, we have built several innovative T-cell products aimed at fighting different tumor types, such as colorectal cancer, pancreatic cancer, ovarian cancer, and through the team led by Eliana Ruggiero, acute myeloid leukemia.
What is the achievement you are proud of the most?
The most important achievements are ahead of me: I want to see our engineered T cells tested in clinical trials. I wish such development to occur here at San Raffaele, where we already have the know-how and the synergy between scientists and clinicians that enable cutting-edge research.
In this context, we identified TCR specific for tumor antigens that are present in different tumors, so that to develop versatile TCR-based therapies that can be used to redirect T cells against – for example –acute myeloid leukemia as well as ovarian cancer. Recently we identified a TCR specific for HER2, an antigen overexpressed by colorectal cancer, breast cancer and other tumor types. Overall, the choice to target widely overexpressed tumor antigens should allow us to contain time, effort and costs, eventually leading to the development of a sustainable cancer immunotherapy.
What would you like to tell a young student who wants to study Hematology?
I chose to specialize in Hematology while I was still studying Medicine at the University. I thought this was the discipline that allowed the most to combine wet research with clinical questions, for the cure of severe diseases.
Nowadays, thanks to outstanding progress in technology, this holds true for many disciplines, but I think that we as hematologists were ahead of time, simply because the blood, but also the bone marrow, are quite accessible samples for investigation and manipulation.
Today, I would still suggest Hematology to a young student starting his career: choose Hematology, and join us in investigating, sometimes answering, and possibly solving, major urgent open questions related to aggressive diseases in need for a cure.
Published on: 18/06/2025
