News
Triple-negative breast cancer: not so negative anymore
Once regarded as one of the most aggressive and difficult-to-treat forms of breast cancer, triple-negative breast cancer (TNBC) is gradually shedding its “negative” label, as research efforts are pointing to new therapeutic options for patients suffering from this tumour.
“TNBC was historically defined by what it lacks,” explains Giampaolo Bianchini, Associate Professor in Medical Oncology at Vita-Salute San Raffaele University and head of the breast oncology unit of the Medical Oncology department at IRCCS San Raffaele Hospital, and “referente ricerca" of the Comprehensive Cancer Center at IRCCS San Raffaele Hospital.
“It is negative for oestrogen receptors, progesterone receptors, and HER2 expression — which means that, for a long time, we had no targeted therapies to offer. Chemotherapy was our only option.”
A new landscape of treatment possibilities
This narrative has changed in recent years. Research has unveiled three major therapeutic pillars that are reshaping the management of TNBC:
- Immunotherapy
- PARP inhibitors
- Antibody-drug conjugates (ADCs)
Immunotherapy has become a cornerstone in the treatment of TNBC both in the early curative setting and in advanced disease with immune checkpoint inhibitors - drugs that unleash a T cell-mediated immune response against the tumor – being the most widely used type of immunotherapy.
When administered in combination with chemotherapy, it helps the immune system recognize and attack tumor cells more effectively.
“Different than melanoma or lung cancer, TNBC is not a highly mutated tumor” says Professor Bianchini. “This means that the immune response alone is not sufficient to effectively act against the tumor mass. This explains why we still need chemotherapy combined with immunotherapy to obtain more effective results”.
PARP inhibitors represent another powerful tool, specifically for patients that carry inherited mutations in BRCA1 and BRCA2 genes. Mutations in these two genes are associated with an increased likelihood of developing breast cancer.
“In addition, such mutations weaken DNA repair mechanisms in tumor cells, making them more vulnerable to the action of PARP inhibitors that normally interfere with those mechanisms. This offers a personalized approach for this group of patients that was unthinkable just a few years ago”, explains the professor.
The third innovation in the treatment of TNBC has come from antibody-drug conjugates, (ADCs), which couple a tumor-targeting antibody with a cytotoxic payload (i.e. a chemotherapeutic agent) via a stable chemical linker. This design allows the chemotherapy drug to be selectively delivered inside cancer cells and on nearby cells, attenuating the systemic exposure to the payload and reducing side effects compared to traditional chemotherapy.
From biology to clinical practice
The development of new treatments beyond chemotherapy is reshaping how health professionals are managing TNBC, but understanding the underlying tumor biology is still crucial to personalizing such therapies and making them more effective.
TNBC is indeed an ecosystem of cell intrinsic properties, such as genomic instability and mutational burden, and cell extrinsic properties that include the tumor microenvironment, the intricate ecosystem of immune and stromal cells that surrounds and interacts with the tumor.
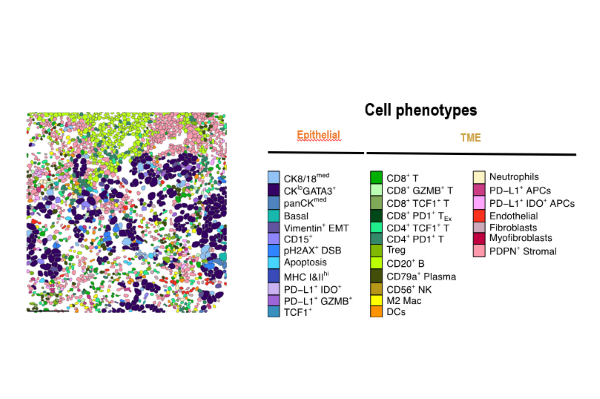

TNBC a heterogeneous disease: an example of a single-cell characterization with spatial distribution by imaging mass cytometry
Characterization of the tumor microenvironment includes understanding how it evolves over time, from the early phases of the disease until the metastatic phase. Furthermore, it’s important for understanding how patients respond to immune checkpoint inhibitors.
In this context, in a landmark Nature publication (2023), Prof. Bianchini’s group used advanced single-cell and spatial imaging techniques to characterize how immune cells behave within TNBC and how they impact cancer response to immune checkpoint inhibitors.
The authors found that the type of immune cells populating the tumor, their spatial organization and their interactions with other cells all affect the characteristics and the response of TNBC to immunotherapy.
Specifically, the presence of proliferating CD8- and TCF1-positive T cells and of MHCII-positive tumor cells, as well as the spatial interaction between the tumor and immune B and T cells expressing granzyme B, are all predictors of a positive response of TNBC to immunotherapy.
“This high-resolution view of the tumor microenvironment helps us to understand why some people with TNBC respond well to immunotherapy while others do not,” he explains. “Identifying these patterns is the first step toward developing predictive biomarkers that could guide treatment decisions in the future.”
While it is important to stress that such tools enabling the mapping of the tumor microenvironment are not yet ready for clinical use, they represent a promising direction for precision oncology as they help to tailor therapies to each patient’s unique biological profile.
An evolving research frontier
The pace of innovation in TNBC research shows no sign of slowing down. ADCs, for example, are currently being explored in numerous combinations and molecular targets across solid tumors. “It’s a whole new world of possibilities,” says Prof. Bianchini. “The key challenge now is to translate these discoveries into durable survival benefits for more patients.”
True innovation stems from continuous education and this is why at San Raffaele, Prof. Bianchini and his colleagues are also committed to training the next generation of oncologists.
“We are witnessing a significant change in how we treat and understand this disease,” he remarks. “It’s essential that young specialists and researchers learn to navigate this complexity, not only through clinical work, but also by understanding the biology that drives these changes.”
In this regard, the Professor often organises post-graduate executive courses dedicated to the training of young health professionals and specifically focused on the innovation in breast cancer biology and therapies.
The next course will take place on November 18, featuring a program rich in presentations, discussions, and round tables on the three therapeutic pillars that are revolutionising the treatment of TNBC.
A message of realism and hope
Despite its persistent aggressiveness, TNBC is no longer the hopeless diagnosis it once was. “I always tell my patients that we’ve moved beyond the stigma of ‘negative,’” concludes Prof. Bianchini. “There is still much work to do, but the progress of recent years gives us reasons for optimism.”
Written by: Laura Celotto
Published on: 31/10/2025
