For Researchers
AIRC 5x1000 | Advanced Therapies for Liver Metastases


Colorectal and pancreatic ductal adenocarcinomas are the second and fourth most common cause of cancer death, respectively.  Patients affected by these cancers often die of liver metastases, which represent the major unmet clinical need for these malignancies.
Patients affected by these cancers often die of liver metastases, which represent the major unmet clinical need for these malignancies.
Based on preliminary data and published observations, we believe that innovative cancer immune gene and cell therapy approaches might overcome the tolerogenic liver microenvironment and represent powerful therapeutic tools for colorectal and pancreatic ductal adenocarcinomas derived liver metastases.
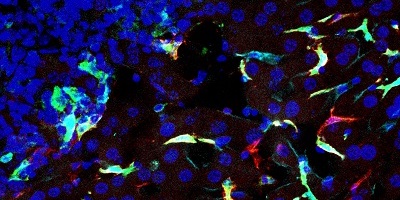
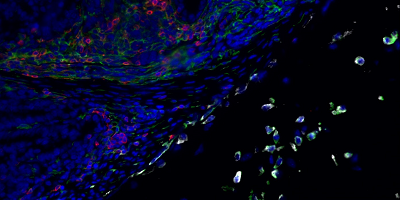
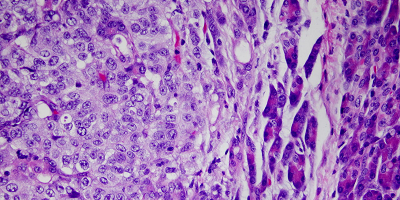
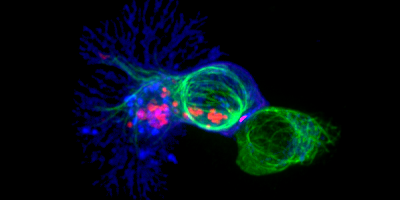
The program gathers together 14 basic research units – working on topics ranging from tumor immunology to gene therapy, from genomics to advanced imaging – and 5 clinical units of IRCCS Ospedale San Raffaele.
It exploits shared state-of-the-art omics, including at single-cell level, advanced imaging, flow cytometry, and histological platforms, and takes advantage of the roadmaps previously established at San Raffaele for the development of gene and cell therapies in other indications.
Download the complete GANTT of the program.
A lay (and up-to-date) description of the program, in Italian, can be found here.
Mission
The ultimate mission of this AIRC translational program is to develop, validate and implement clinical testing of new advanced therapy medicinal products (ATMP), based on immune gene and cell therapies, for liver metastases that arise from colorectal and pancreatic ductal adenocarcinomas.
Experimental Design
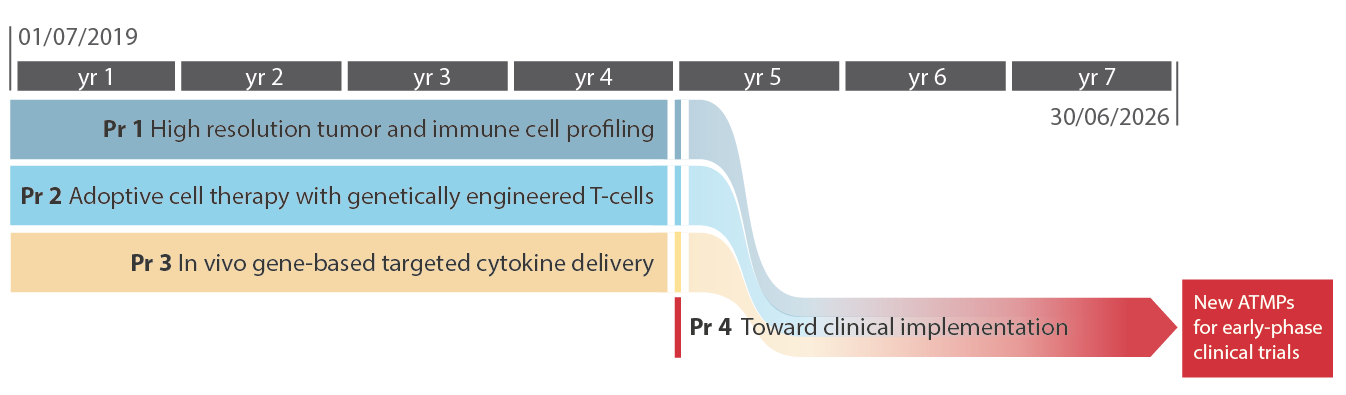
The proposal spans 7 years and is built on 4 highly integrated programs:
- Program 1 (coordinators: Paolo Dellabona; Luca Aldrighetti) aims at unraveling - using biological samples collected through an observational clinical study (LiMeT) - the immune infiltrate and cancer cell profiles in liver metastasis from colorectal and pancreatic ductal adenocarcinomas, to identify tumor-associated antigens and immune suppressive and regulatory pathways to be targeted in these two diseases.
The results of this discovery program will progressively shape two independent but potentially combinatorial translational programs (Program 2 and 3), tackling hepatic metastasis by rational and targeted modification of the immune effectors within the tumor microenvironment, through state-of-the-art cell therapies and gene transfer/editing tools. - Program 2 (coordinators: Chiara Bonini; Massimo Falconi) aims at harnessing T cells with genetic tools that allow their retargeting against cancer cells while ensuring resistance to the immunosuppressive tumor microenvironment.
- Program 3 (coordinators: Luigi Naldini; Michele Reni) will modify the immunosuppressive microenvironment through novel targeted in vivo gene therapies aimed at enhancing spontaneous innate and adaptive immunity against the tumor and the efficacy of exogenous T cell responses. Program 2- and 3-generated ATMPs will be tested as single therapies as well as in combination, to exploit synergistic effects.
- Data and therapeutic candidates generated in the first 4 years by the three concurring programs (1-3) will pave the way for the successive phase, in which Program 4, through the integrated work of all the participating units and the support of intramural facilities, will stringently assess the most promising ATMPs for efficacy and toxicity, select the best-performing products for further development and prioritize the top lead(s) for early phase clinical trials launched towards the end of the funding period.
Achievements
By matched and multi-level analyses of patients’ biological samples, Program 1 has:
- identified different immunoregulatory receptors and molecules involved in the immune suppression and exhaustion distinctive of hepatic metastases (first milestone achieved);
- selected several tumor antigens eligible for CAR development and/or TCR redirection (second milestone achieved).
The institutional biobank (Center of Biological Resources, CRB) collects several biological specimens from patients enrolled in LiMeT clinical study (almost 1000 patients since its authorization in November 2019), including follow-up samples, allowing cross-sectional and longitudinal analyses planned by Program 1.
Patient clinical data at enrollment and during follow-up are recorded in a GDPR-compliant intramural database (Cohort Genomics Platform, CGP), providing clinical correlates of experimental results from Program 1.
By an optimized pipeline for T cell gene editing, Program 2 has established the genetic deletion of those immunoregulatory receptors identified by Program 1. Moreover, new CAR- and TCR-T cell products have been generated, tested for specific antitumor activity, and are now under safety validation. Three best-performing T cell products have been selected for further development towards clinical use (third milestone achieved).
Program 3 has designed and produced different peptide-based formulations for tumor/stroma targeting and a tunable lentivirus(LV)-based platform for liver-targeted cytokine delivery, by which a candidate antitumoral cytokine has been selectively expressed in liver metastases of tumor-bearing mice, exerting promising therapeutic effects in the absence of systemic toxicity. One peptide-based and one LV-based approach have been selected for clinical translation (fourth milestone achieved). In particular, one of them will be further developed in combination with one T cell product selected by Program 2 to widen and synergize their clinical applications (fifth milestone achieved).
Different ex-vivo and in vivo models have been developed and shared by the AIRC5x1000 consortium, to recapitulate hepatic metastases of colorectal and pancreatic ductal adenocarcinomas. They were instrumental for efficacy and toxicity studies by Programs 2 and 3.
The Program passed the 3rd- and 5th-year midterm reviews, in March 2022 and 2024, respectively, obtaining enthusiastic consensus endorsement by a panel of 9 international experts in translational oncology, clinical immunology and genetics.
Program 4 is in full swing, with the five selected ATMPs (the “TOP 5”) progressing through biodistribution and toxicity studies, while protocols for GMP-compliant manufacturing, scaled-up production and quality control testing are being set-up with the support of intramural facilities and external collaborations.
Among the “TOP 5” selected ATMPs, the CAR-T cell product developed by Program 2 for metastatic colorectal cancer has emerged as the most advanced candidate for clinical translation. As a result, during the final year of the project, the majority of activities and resources across the consortium are being directed toward enabling a first-in-human trial with this product, with the goal of submitting the clinical trial application by the end of the funding period.
Importantly, all five therapeutic products selected by the program for future clinical translation are protected by patents, and several have already secured external funding sources (e.g., EXTEND funding for the two therapies targeting pancreatic ductal adenocarcinoma), further underscoring the translational potential of these products. This ensures their continued development toward clinical use independently of the resources allocated within the current research program.
In parallel, several strategic actions have been undertaken to facilitate the next clinical development steps and address regulatory challenges specific to ATMPs:
a. a Clinical Translation Working Group (CT-WG), coordinated by Fabio Ciceri, was established to bring together internal and external stakeholders — including developers of the five ATMPs, clinicians, members of the Process Development and GLP laboratories, and CMC experts — to design the upcoming first-in-human trial(s);
b. two leading clinical experts have joined the program’s advisory board to support strategic planning and operational decisions;
c. the program has been embedded within the intramural Comprehensive Cancer Center (OSR-CCC) and is now connected with broader European networks and initiatives focused on advanced therapies, such as the EU-funded JOIN4ATMP project.
Last update: 18.07.2025
SCIENTIFIC EXCELLENCE: AWARDS, HONOURS & GRANTS
The national and international recognitions awarded to both junior and senior members of the program — including prestigious honours, scientific awards, and competitive research grants — are a testament to the outstanding quality of the participants and the high-impact nature of the research conducted. From early-career investigators to leading principal scientists, these achievements highlight the strength and visibility of the program at both institutional and international levels. Below are selected examples (award/grant, recipient):
9th edition of the Oncology Research Prize 2025, Alessia Potenza
DKMS Mechtild Harf Science Award 2025, Chiara Bonini
Outstanding Achievement Award 2024 from the European Society of Gene and Cell Therapy, Chiara Bonini
ERC Synergy Grant 2023, Valeria Fumagalli
Last update: 09.07.2025
Prof. Dr. Hana Algül
Technische Universität München, Munich
Prof. Dr. Dirk Busch
Technische Universität München, Munich
Dr. Luca Gianni
Gianni Bonadonna Foundation, Milan
Prof. Concetta Quintarelli
Bambino Gesù Children's Hospital, Rome
Prof. Salvatore Siena
Niguarda Hospital, Milan
Prof. Francesco Sclafani (since February 2023)
Institut Jules Bordet, Brussels
Prof. Nicola Valeri (former)
The Institute of Cancer Research, London
Imperial College London, London
The program gathers together 5 clinical units of IRCCS Ospedale San Raffaele:
- Hepatobiliary surgery - Head: Francesca Ratti
- Anatomic pathology and histopathology - Head: Claudio Doglioni
- Pancreatic surgery - Head: Massimo Falconi
- Oncology - Head: Michele Reni
- Diagnostic imaging – Head: Francesco De Cobelli
Spatiotemporal liver dynamics shape hepatocellular heterogeneity and impact in vivo gene engineering. Milani M, Starinieri F, Beretta S, Monti M, Canepari C, Marabotti F, Zambrano S, Mazza D, Fabiano A, Simoni C, Cammarota E, Volpin M, Bortolussi G, Russo F, Biffi M, Genua M, Degl'Innocenti S, Sanvito F, Ostuni R, Muro AF, Montini E, Moalli F, Iannacone M, Merelli I, Cantore A. J Hepatol. 2025 Dec;83(6):1392-1409. doi: 10.1016/j.jhep.2025.06.018.
IL-1 family members as regulators of lymphoid type-2 immunity in cancer. Roma S, Colombo F, De Monte L, Protti MP. Semin Immunol. 2025 Nov 28;81:102005. doi: 10.1016/j.smim.2025.102005.
The co-inhibitory receptor TIGIT promotes tissue-protective functions in T cells. Panetti C, Daetwyler R, Moncsek A, Patikas N, Agrafiotis A, Tang A, Andreata F, Fumagalli V, De Lima J, Wen L, King CG, Vasanthakumar A, Iannacone M, Kallies A, Yermanos A, Hemberg M, Joller N. Nat Immunol. 2025 Nov;26(11):2074-2085. doi: 10.1038/s41590-025-02300-w.
Scientific and medical evidence informing expansion of hepatitis B treatment guidelines. Kennedy PT, Allweiss L, Bertoletti A, Cornberg M, Gehring AJ, Guidotti LG, Kerth HA, Lemoine M, Levrero M, Lim SG, Tavis JE, Testoni B, Tu T; International Coalition to Eliminate HBV. Lancet Gastroenterol Hepatol. 2025 Oct;10(10):941-951. doi: 10.1016/S2468-1253(25)00053-6.
From Histology to High-Resolution Mapping: The Rise of Spatial Omics in Immunology. De Giovanni M, Inverso D, Iannacone M. Eur J Immunol. 2025 Oct;55(10):e70073. doi: 10.1002/eji.70073.
A rapid and robust luciferase-based reporter system to assess SARS-CoV-2 protease activity. Lucini Paioni A, Donnici L, Nodari R, Longo Minnolo M, Ferraro A, Alberico A, Brindisi M, Mejías Pérez E, Keppler OT, Summa V, Guidotti LG, Albanese M, De Francesco R. Virology. 2025 Oct;611:110659. doi: 10.1016/j.virol.2025.110659.
CD4+ T cells license Kupffer cells to reverse CD8+ T cell dysfunction induced by hepatocellular priming. Venzin V, Beccaria CG, Perucchini C, Delfino P, Bono EB, Giustini L, Moalli F, Grillo M, Fumagalli V, Laura C, Di Lucia P, Reinhard K, Petschenka J, Omokoko TA, Celant A, Ottolini S, Kawashima K, Ravà M, De Giovanni M, Inverso D, Kuka M, Kennedy PTF, Guilliams M, Casorati G, Pedica F, Ponzoni M, Şahin U, Le Bert N, Bertoletti A, Vascotto F, Guidotti LG, Iannacone M. Nat Immunol. 2025 Aug;26(8):1352-1366. doi: 10.1038/s41590-025-02199-3.
Prognostic Validation of Perineural Invasion Severity Score in Pancreatic Cancer: A Prospective Study. Gasparini G, Crippa S, Lena MS, Belfiori G, Aleotti F, Di Salvo F, Pellegatta M, Tamburrino D, Partelli S, Pecorelli N, Cangi MG, Taveggia C, Reni M, Doglioni C, Falconi M. Ann Surg. 2025 Aug 26. doi: 10.1097/SLA.0000000000006920.
Decoration of gold nanoparticles with glycopeptides leads to a lower cellular uptake and liver retention. Soliman MG, Alarcon JF, Lüdtke TU, Violatto MB, Dobricic M, Cordiglieri C, Corbelli A, Fiordaliso F, Sitia G, O'Donnell JS, Spencer DIR, Moya S, Bigini P, Monopoli MP. Nanoscale Adv. 2025 Aug 12;7(18):5784-5798. doi: 10.1039/d5na00464k.
Targeting HBV with RNA interference: Paths to cure. Iannacone M, Beccaria CG, Allweiss L, Lucifora J, Tavis JE, Gehring AJ, Dandri M.Sci Transl Med. 2025 Jul 2;17(805):eadv3678. doi: 10.1126/scitranslmed.adv3678.
Systemic delivery of cadherin 17-specific CAR T cells allows effective and safe targeting of colorectal cancer liver metastases. Greco B, El Khoury R, Balestrieri C, Sirini C, Machado A, De Girardi F, De Rossi L, Botrugno OA, Giovannoni G, Falcone L, Camisa B, Tiziano E, Palmisano K, Campodonico E, Moresco MA, Perani L, Bergamini A, Facoetti A, Ungaro F, Tonon G, Donnadieu E, Doglioni C, Ciceri F, Bonini C, Casucci M. Sci Transl Med. 2025 May 28;17(800):eadr1928. doi: 10.1126/scitranslmed.adr1928.
T-cell-derived IFN-γ suppresses T follicular helper cell differentiation and antibody responses. Sala E, Nelli M, Laura C, Di Lucia P, Beccaria CG, Bono EB, Mangione M, Marotta D, Sperto V, Grillo M, Giustini L, Tosi F, Nie J, Kim D, Furiato G, Malpighi C, Consolo E, Becher B, David E, Cohen M, Giladi A, Amit I, Bosselut R, Guidotti LG, Iannacone M, Kuka M. T-cell-derived IFN-γ suppresses T follicular helper cell differentiation and antibody responses. EMBO J. 2025 May;44(9):2400-2423. doi: 10.1038/s44318-025-00414-3.
Reprogramming liver metastasis-associated macrophages toward an anti-tumoral phenotype through enforced miR-342 expression. Bresesti C, Carito E, Notaro M, Giacca G, Breggion S, Kerzel T, Mercado CM, Beretta S, Monti M, Merelli I, Canu T, Naldini L, Squadrito ML. Cell Rep. 2025 May 27;44(5):115592. doi: 10.1016/j.celrep.2025.115592.
Anatomically resectable versus biologically borderline resectable pancreatic cancer definition: refining the border beyond anatomical criteria and biological aggressiveness. Belfiori G, De Stefano F, Tamburrino D, Gasparini G, Aleotti F, Camisa PR, Arcangeli C, Schiavo Lena M, Pecorelli N, Palumbo D, Partelli S, De Cobelli F, Reni M, Crippa S, Falconi M. BJS Open. 2025 May 7;9(3):zraf033. doi: 10.1093/bjsopen/zraf033.
Functional Polarization of Liver Macrophages by Glyco Gold Nanoparticles. Fernandez Alarcon J, Perez Schmidt P, Panini N, Caruso F, Violatto MB, Sukubo NG, Martinez-Serra A, Ekalle-Soppo CB, Morelli A, Moscatiello GY, Grasselli C, Corbelli A, Fiordaliso F, Kelk J, Petrosilli L, d'Orazio G, Mateu Ferrando R, Verdaguer Ferrer A, Fornaguera C, Lay L, Fumagalli S, Recchia S, Monopoli MP, Polito L, Bigini P, Sitia G. Adv Sci (Weinh). 2025 Apr;12(16):e2407458. doi: 10.1002/advs.202407458.
In vivo armed macrophages curb liver metastasis through tumor-reactive T-cell rejuvenation. Notaro M, Borghetti M, Bresesti C, Giacca G, Kerzel T, Mercado CM, Beretta S, Monti M, Merelli I, Iaia S, Genua M, Annoni A, Canu T, Cristofori P, Degl'Innocenti S, Sanvito F, Rancoita PMV, Ostuni R, Gregori S, Naldini L, Squadrito ML. Nat Commun. 2025 Apr 11;16(1):3471. doi: 10.1038/s41467-025-58369-2
Blockade of αvβ6 and αvβ8 integrins with a chromogranin A-derived peptide inhibits TGFβ activation in tumors and suppresses tumor growth. Gasparri AM, Pocaterra A, Colombo B, Taiè G, Gnasso C, Gori A, Pozzi F, Smith A, Magni F, Ugolini A, Doglio M, Bonini MC, Mondino A, Corti A, Curnis F. J Exp Clin Cancer Res. 2025 Mar 8;44(1):88. doi: 10.1186/s13046-025-03352-4.
Unlocking CD8+ T cell potential in chronic hepatitis B virus infection. Fumagalli V, Iannacone M. Nat Rev Gastroenterol Hepatol. 2025 Feb;22(2):92-93. doi: 10.1038/s41575-024-01015-x.
Functional Polarization of Liver Macrophages by Glyco Gold Nanoparticles. Fernandez Alarcon J, Perez Schmidt P, Panini N, Caruso F, Violatto MB, Sukubo NG, Martinez-Serra A, Ekalle-Soppo CB, Morelli A, Moscatiello GY, Grasselli C, Corbelli A, Fiordaliso F, Kelk J, Petrosilli L, d'Orazio G, Mateu Ferrando R, Verdaguer Ferrer A, Fornaguera C, Lay L, Fumagalli S, Recchia S, Monopoli MP, Polito L, Bigini P, Sitia G. Adv Sci (Weinh). 2025 Feb 14:e2407458. doi: 10.1002/advs.202407458.
CAR T cells in solid tumors and metastasis: paving the way forward. Sirini C, De Rossi L, Moresco MA, Casucci M. Cancer Metastasis Rev. 2024 Dec;43(4):1279-1296. doi: 10.1007/s10555-024-10213-7.
A pilot study of chlorambucil in pre-treated metastatic pancreatic adenocarcinoma patients bearing germline BRCA or other DNA damage repair system variants. Carconi C, Bosi C, Scartozzi M, Cergnul M, Cinausero M, Faloppi L, Garajova I, Lonardi S, Pecora I, Pisanu L, Spadi R, Spallanzani A, Peretti U, Macchini M, Orsi G, Reni M. Pancreatology. 2024 Nov;24(7):1066-1072. doi: 10.1016/j.pan.2024.09.006.
CAR-redirected natural killer T cells demonstrate superior antitumor activity to CAR-T cells through multimodal CD1d-dependent mechanisms. Zhou X, Wang Y, Dou Z, Delfanti G, Tsahouridis O, Pellegry CM, Zingarelli M, Atassi G, Woodcock MG, Casorati G, Dellabona P, Kim WY, Guo L, Savoldo B, Tsagaratou A, Milner JJ, Metelitsa LS, Dotti G. Nat Cancer. 2024 Nov;5(11):1607-1621. doi: 10.1038/s43018-024-00830-0.
Intrahepatic immunity to hepatitis B virus. Venzin V, Beccaria CG, Andreata F, Fumagalli V, Iannacone M. J Hepatol. 2024 Nov;81(5):911-913. doi: 10.1016/j.jhep.2024.05.029.
Intercellular nanotube-mediated mitochondrial transfer enhances T cell metabolic fitness and antitumor efficacy. Baldwin JG, Heuser-Loy C, Saha T, Schelker RC, Slavkovic-Lukic D, Strieder N, Hernandez-Lopez I, Rana N, Barden M, Mastrogiovanni F, Martín-Santos A, Raimondi A, Brohawn P, Higgs BW, Gebhard C, Kapoor V, Telford WG, Gautam S, Xydia M, Beckhove P, Frischholz S, Schober K, Kontarakis Z, Corn JE, Iannacone M, Inverso D, Rehli M, Fioravanti J, Sengupta S, Gattinoni L. Cell. 2024 Nov 14;187(23):6614-6630.e21. doi: 10.1016/j.cell.2024.08.029.
Unlocking CD8+ T cell potential in chronic hepatitis B virus infection. Fumagalli V, Iannacone M. Nat Rev Gastroenterol Hepatol. 2024 Nov 18. doi: 10.1038/s41575-024-01015-x.
Understanding local immunity to enable regionalized medicine. De Giovanni M, Inverso D, Iannacone M. EMBO J. 2024 Oct 9. doi: 10.1038/s44318-024-00255-6.
Futility of Up-Front Resection for Anatomically Resectable Pancreatic Cancer. Crippa S, Malleo G, Mazzaferro V, Langella S, Ricci C, Casciani F, Belfiori G, Galati S, D'Ambra V, Lionetto G, Ferrero A, Casadei R, Ercolani G, Salvia R, Falconi M, Cucchetti A. JAMA Surg. 2024 Oct 1;159(10):1139-1147. doi: 10.1001/jamasurg.2024.2485.
A preclinical mouse model of hepatic metastasis to instruct effective treatment modalities. Pocaterra A, Citro A, Gnasso C, Canu T, Tosi A, Rosato A, Esposito A, Piemonti L, Mondino A. Methods Cell Biol. 2024;190:133-150. doi: 10.1016/bs.mcb.2024.07.008.
VisualZoneR: A computational protocol to identify compartmental zones from single-cell spatial transcriptomics using R. Kerzel T, Beretta S, Naldini L, Squadrito ML. STAR Protoc. 2024 Sep 20;5(3):103196. doi: 10.1016/j.xpro.2024.103196.
Establishment of a Transplantation Model of PDAC-Derived Liver Metastases. Ferrara B, Dugnani E, Citro A, Schiavo Lena M, Marra P, Camisa PR, Policardi M, Canu T, Esposito A, Doglioni C, Piemonti L. Ann Surg Oncol. 2024 Sep;31(9):6138-6146. doi: 10.1245/s10434-024-15514-3.
Antibodies and complement are key drivers of thrombosis. Stark K, Kilani B, Stockhausen S, Busse J, Schubert I, Tran TD, Gaertner F, Leunig A, Pekayvaz K, Nicolai L, Fumagalli V, Stermann J, Stephan F, David C, Müller MB, Heyman B, Lux A, da Palma Guerreiro A, Frenzel LP, Schmidt CQ, Dopler A, Moser M, Chandraratne S, von Brühl ML, Lorenz M, Korff T, Rudelius M, Popp O, Kirchner M, Mertins P, Nimmerjahn F, Iannacone M, Sperandio M, Engelmann B, Verschoor A, Massberg S. Immunity. 2024 Sep 10;57(9):2140-2156.e10. doi: 10.1016/j.immuni.2024.08.007.
Therapeutic potential of co-signaling receptor modulation in hepatitis B. Andreata F, Laura C, Ravà M, Krueger CC, Ficht X, Kawashima K, Beccaria CG, Moalli F, Partini B, Fumagalli V, Nosetto G, Di Lucia P, Montali I, Garcia-Manteiga JM, Bono EB, Giustini L, Perucchini C, Venzin V, Ranucci S, Inverso D, De Giovanni M, Genua M, Ostuni R, Lugli E, Isogawa M, Ferrari C, Boni C, Fisicaro P, Guidotti LG, Iannacone M. Cell. 2024 Jul 25;187(15):4078-4094.e21. doi: 10.1016/j.cell.2024.05.038.
Anakinra-Loaded Sphingomyelin Nanosystems Modulate In Vitro IL-1-Dependent Pro-Tumor Inflammation in Pancreatic Cancer. Abal-Sanisidro M, De Luca M, Roma S, Ceraolo MG, de la Fuente M, De Monte L, Protti MP. Int J Mol Sci. 2024 Jul 24;25(15):8085. doi: 10.3390/ijms25158085.
LIM-domain-only 4 (LMO4) enhances CD8+ T-cell stemness and tumor rejection by boosting IL-21-STAT3 signaling. Schelker RC, Fioravanti J, Mastrogiovanni F, Baldwin JG, Rana N, Li P, Chen P, Vadász T, Spolski R, Heuser-Loy C, Slavkovic-Lukic D, Noronha P, Damiano G, Raccosta L, Maggioni D, Pullugula S, Lin JX, Oh J, Grandinetti P, Lecce M, Hesse L, Kocks E, Martín-Santos A, Gebhard C, Telford WG, Ji Y, Restifo NP, Russo V, Rehli M, Herr W, Leonard WJ, Gattinoni L. Signal Transduct Target Ther. 2024 Aug 9;9(1):199. doi: 10.1038/s41392-024-01915-z.
Priming and Maintenance of Adaptive Immunity in the Liver. Kawashima K, Andreata F, Beccaria CG, Iannacone M. Annu Rev Immunol. 2024 Jun;42(1):375-399. doi: 10.1146/annurev-immunol-090122-041354.
Very Early Recurrence After Curative Resection for Pancreatic Ductal Adenocarcinoma: Proof of Concept for a "Biological R2 Definition". Belfiori G, Crippa S, Pagnanelli M, Gasparini G, Aleotti F, Camisa PR, Partelli S, Pecorelli N, De Stefano F, Schiavo Lena M, Palumbo D, Tamburrino D, Reni M, Falconi M. Ann Surg Oncol. 2024 Jun;31(6):4084-4095. doi: 10.1245/s10434-024-15105-2.
GPR126 is a specifier of blood-brain barrier formation in the mouse central nervous system. Kakogiannos N, Scalise AA, Martini E, Maderna C, Benvenuto AF, D'Antonio M, Carmignani L, Magni S, Gullotta GS, Lampugnani MG, Iannelli F, Beznoussenko GV, Mironov AA, Cerutti C, Bentley K, Philippides A, Zanardi F, Bacigaluppi M, Sigismund S, Bassani C, Farina C, Martino G, De Giovanni M, Dejana E, Iannacone M, Inverso D, Giannotta M. J Clin Invest. 2024 Jun 6;134(15):e165368. doi: 10.1172/JCI165368.
Antibody-independent protection against heterologous SARS-CoV-2 challenge conferred by prior infection or vaccination. Fumagalli V, Ravà M, Marotta D, Di Lucia P, Bono EB, Giustini L, De Leo F, Casalgrandi M, Monteleone E, Mouro V, Malpighi C, Perucchini C, Grillo M, De Palma S, Donnici L, Marchese S, Conti M, Muramatsu H, Perlman S, Pardi N, Kuka M, De Francesco R, Bianchi ME, Guidotti LG, Iannacone M. Nat Immunol. 2024 Apr;25(4):633-643. doi: 10.1038/s41590-024-01787-z.
TIM-3, LAG-3, or 2B4 gene disruptions increase the anti-tumor response of engineered T cells. Cianciotti BC, Magnani ZI, Ugolini A, Camisa B, Merelli I, Vavassori V, Potenza A, Imparato A, Manfredi F, Abbati D, Perani L, Spinelli A, Shifrut E, Ciceri F, Vago L, Di Micco R, Naldini L, Genovese P, Ruggiero E, Bonini C. Front Immunol. 2024 Feb 29;15:1315283. doi: 10.3389/fimmu.2024.1315283.
The interplay of drug therapeutics and immune responses to SARS-CoV-2. Fumagalli V, Iannacone M. Cell Mol Immunol. 2024 Feb;21(2):197-200. doi: 10.1038/s41423-023-01098-7.
A novel aminopeptidase N/CD13 inhibitor selectively targets an endothelial form of CD13 after coupling to proteins. Anderluzzi G, Ghitti M, Gasparri AM, Taiè G, Sacchi A, Gori, A, Andolfo A, Pozzi F, Musco G, Curnis F^ ,Corti A^ (^=co-corresponding author). Cell Mol Life Sci. 2024; 81(1): 68. Published online 2024 Jan 30. doi: 10.1007/s00018-023-05102-1
Deterministic reprogramming of neutrophils within tumors. Ng MSF, Kwok I, Tan L, Shi C, Cerezo-Wallis D, Tan Y, Leong K, Calvo GF, Yang K, Zhang Y, Jin J, Liong KH, Wu D, He R, Liu D, Teh YC, Bleriot C, Caronni N, Liu Z, Duan K, Narang V, Ballesteros I, Moalli F, Li M, Chen J, Liu Y, Liu L, Qi J, Liu Y, Jiang L, Shen B, Cheng H, Cheng T, Angeli V, Sharma A, Loh YH, Tey HL, Chong SZ, Iannacone M, Ostuni R, Hidalgo A, Ginhoux F, Ng LG. Science. 2024 Jan 12;383(6679):eadf6493. doi: 10.1126/science.adf6493.
Harnessing lentiviral vectors for in vivo gene therapy of liver metastases. Giacca G, Naldini L, Squadrito ML. Clin Transl Med. 2024 Jan;14(1):e1542. doi: 10.1002/ctm2.1542.
CD8 cis-targeted IL-2 drives potent antiviral activity against hepatitis B virus. Andreata F, Moynihan KD, Fumagalli V, Di Lucia P, Pappas DC, Kawashima K, Ni I, Bessette PH, Perucchini C, Bono E, Giustini L, Nguyen HC, Chin SM, Yeung YA, Gibbs CS, Djuretic I, Iannacone M. Sci Transl Med. 2024 Jan 10;16(729):eadi1572. doi: 10.1126/scitranslmed.adi1572.
A fluorescent reporter model for the visualization and characterization of TDC. Fiore A, Sala E, Laura C, Riba M, Nelli M, Fumagalli V, Oberrauch F, Mangione M, Cristofani C, Provero P, Iannacone M, Kuka M. Eur J Immunol. 2023 Dec;53(12):e2350529. doi: 10.1002/eji.202350529.
Cure Probabilities After Resection Of Pancreatic Ductal Adenocarcinoma: A Multi-Institutional Analysis Of 2554 Patients. Crippa S, Malleo G, Langella S, Ricci C, Casciani F, Belfiori G, Galati S, Ingaldi C, Lionetto G, Ferrero A, Casadei R, Ercolani G, Salvia R, Falconi M, Cucchetti A.Ann Surg. 2023 Dec 4. doi: 10.1097/SLA.0000000000006166.
Harnessing T cell exhaustion and trogocytosis to isolate patient-derived tumor-specific TCR. Manfredi F, Stasi L, Buonanno S, Marzuttini F, Noviello M, Mastaglio S, Abbati D, Potenza A, Balestrieri C, Cianciotti BC, Tassi E, Feola S, Toffalori C, Punta M, Magnani Z, Camisa B, Tiziano E, Lupo-Stanghellini MT, Branca RM, Lehtiö J, Sikanen TM, Haapala MJ, Cerullo V, Casucci M, Vago L, Ciceri F, Bonini C, Ruggiero E. Sci Adv. 2023 Dec;9(48):eadg8014. doi: 10.1126/sciadv.adg8014.
Long-term retention of gold nanoparticles in the liver is not affected by their physicochemical characteristics. Alarcon JF, Soliman M, Ludtke T, Clemente E, Dobricic M, Violatto MB, Corbelli A, Fiordaliso F, Cordiglieri C, Laura Talamini, Sitia G, Moya S, Bigini Pand Monopoli MP. Nanoscale. 2023 Apr 25.
Variations in Biodistribution and Acute Response of Differently Shaped Titania Nanoparticles in Healthy Rodents. Violatto MB, Sitia G, Talamini L, Morelli M, Lan Tran N, Zhang Q, Masood A, Pelaz B, Chakraborty I, Cui D, Parak WJ, Salmona M, Bastús NG, Puntes V and Bigini P. Nanomaterials 2023, 13, 1174.
In vivo macrophage engineering reshapes the tumor microenvironment leading to eradication of liver metastases. Kerzel T, Giacca G, Beretta S, Bresesti C, Notaro M, Scotti GM, Balestrieri C, Canu T, Redegalli M, Pedica F, Genua M, Ostuni R, Kajaste-Rudnitski A, Oshima M, Tonon G, Merelli I, Aldrighetti L, Dellabona P, Coltella N, Doglioni C, Rancoita PMV, Sanvito F, Naldini L, Squadrito ML. Cancer Cell. 2023 Nov 13;41(11):1892-1910.e10. doi: 10.1016/j.ccell.2023.09.014.
A stapled chromogranin A-derived peptide homes in on tumors that express αvβ6 or αvβ8 integrins. Monieri M, Rainone P, Sacchi A, Gori A, Gasparri AM, Coliva A, Citro A, Ferrara B, Policardi M, Valtorta S, Pocaterra A, Alfano M, Sheppard D, Piemonti L, Moresco RM, Corti A*, Curnis F*. Int J Biol Sci. 2023; 19(1):156-166. doi:10.7150/ijbs.76148. (*Corresponding Authors)
IL-1β+ macrophages fuel pathogenic inflammation in pancreatic cancer. Caronni N, La Terza F, Vittoria FM, Barbiera G, Mezzanzanica L, Cuzzola V, Barresi S, Pellegatta M, Canevazzi P, Dunsmore G, Leonardi C, Montaldo E, Lusito E, Dugnani E, Citro A, Ng MSF, Schiavo Lena M, Drago D, Andolfo A, Brugiapaglia S, Scagliotti A, Mortellaro A, Corbo V, Liu Z, Mondino A, Dellabona P, Piemonti L, Taveggia C, Doglioni C, Cappello P, Novelli F, Iannacone M, Ng LG, Ginhoux F, Crippa S, Falconi M, Bonini C, Naldini L, Genua M, Ostuni R. Nature. 2023 Nov;623(7986):415-422. doi: 10.1038/s41586-023-06685-2.
Epithelial ovarian cancer is infiltrated by activated effector T cells co-expressing CD39, PD-1, TIM-3, CD137 and interacting with cancer cells and myeloid cells. Tassi E, Bergamini A, Wignall J, Sant'Angelo M, Brunetto E, Balestrieri C, Redegalli M, Potenza A, Abbati D, Manfredi F, Cangi MG, Magliacane G, Scalisi F, Ruggiero E, Maffia MC, Trippitelli F, Rabaiotti E, Cioffi R, Bocciolone L, Candotti G, Candiani M, Taccagni G, Schultes B, Doglioni C, Mangili G, Bonini C. Front Immunol. 2023 Oct 4;14:1212444. doi: 10.3389/fimmu.2023.1212444.
Harnessing the reverse cholesterol transport pathway to favor differentiation of monocyte-derived APCs and antitumor responses. Raccosta L, Marinozzi M, Costantini S, Daniela Maggioni, Ferreira LM, Corna G, Zordan P, Sorice A, Farinello D, Bianchessi S, Riba M, Lazarevic D, Provero P, Mack M, Bondanza A, Nalvarte I, Gustafsson J-A, Ranzani V, De Sanctis F, Ugel S, Baron S, Lobaccaro J- A, Pontini L, Pacciarini M, Traversari C, Pagani M, Bronte V, Sitia G, Antonson P, Brendolan A, Budillon A & Russo V. Cell Death Dis. 14, 129 (2023).
Revealing and harnessing CD39 for the treatment of colorectal cancer and liver metastases by engineered T cells. Potenza A*, Balestrieri C*, Spiga M, Albarello L, Pedica F, Manfredi F, Cianciotti BC, De Lalla C, Botrugno OA, Faccani C, Stasi L, Tassi E, Bonfiglio S, Scotti GM, Redegalli M, Biancolini D, Camisa B, Elena Tiziano, Sirini C, Casucci M, Iozzi C, Abbati D, Simeoni F, Lazarevic D, Elmore U, Fiorentini G, Di Lullo G, Casorati G, Doglioni D, Tonon G, Dellabona P, Rosati R, Aldrighetti L, Ruggiero E^ and Bonini C^ (*= equal contribution, ^=co-last author). Gut. 2023 Oct;72(10):1887-1903. doi: 10.1136/gutjnl-2022-328042.
Genome Editing in Engineered T Cells for Cancer Immunotherapy. Bonini C, Chapuis AG, Hudecek M, Guedan S, Magnani C, Qasim W. Hum Gene Ther. 2023 Sep;34(17-18):853-869. doi: 10.1089/hum.2023.128.
Pro-tumor Tfh2 cells induce detrimental IgG4 production and PGE2-dependent IgE inhibition in pancreatic cancer. De Monte L, Clemente F, Ruggiero E, Pini R, Ceraolo MG, Schiavo Lena M, Balestrieri C, Lazarevic D, Belfiori G, Crippa S, Balzano G, Falconi M, Doglioni C, Bonini C, Reni M, Protti MP. EBioMedicine. 2023 Sep 28;97:104819. doi: 10.1016/j.ebiom.2023.104819.
Cyclophosphamide maintenance to extend combination chemotherapy-free interval in metastatic pancreatic ductal adenocarcinoma. Reni M, Peretti U, Macchini M, Orsi G, Militello A, Briccolani A, Falconi M, Cascinu S. Dig Liver Dis. 2023 Aug 14:S1590-8658(23)00784-3. doi: 10.1016/j.dld.2023.07.033.
Exploring the optimal therapeutic management of stage ypIA pancreatic ductal adenocarcinoma patients in the era of primary chemotherapy. Macchini M, Belfiori G, Crippa S, Orsi G, Gasparini G, Tamburrino D, Partelli S, Schiavo Lena M, Palumbo D, De Cobelli F, Falconi M, Reni M. Dig Liver Dis. 2023 Jul 15:S1590-8658(23)00758-2. doi: 10.1016/j.dld.2023.07.006.
Primary Mouse Invariant Natural Killer T (iNKT) Cell Purification and Transduction. Delfanti G, Dellabona P, Casorati G. Bio Protoc. 2023 Jul 5;13(13):e4707. doi: 10.21769/BioProtoc.4707.
Cytomegalovirus-specific T cells restricted for shared and donor human leukocyte antigens differentially impact on cytomegalovirus reactivation risk after allogeneic hematopoietic stem cell transplantation. Tassi E, Noviello M, De Simone P, Lupo-Stanghellini MT, Doglio M, Serio F, Abbati D, Beretta V, Valtolina V, Oliveira G, Racca S, Campodonico E, Ruggiero E, Clerici D, Giglio F, Lorentino F, Dvir R, Xue E, Farina F, Oltolini C, Manfredi F, Vago L, Corti C, Bernardi M, Clementi M, Brix L, Ciceri F, Peccatori J, Greco R, Bonini C. Haematologica. 2023 Jun 1;108(6):1530-1543. doi: 10.3324/haematol.2022.280685.
Charting granulopoietic disturbances in sepsis. Barouni RM, Ostuni R. Nat Immunol. 2023 May;24(5):746-748. doi: 10.1038/s41590-023-01495-0.
Nirmatrelvir treatment of SARS-CoV-2-infected mice blunts antiviral adaptive immune responses. Fumagalli V, Di Lucia P, Ravà M, Marotta D, Bono E, Grassi S, Donnici L, Cannalire R, Stefanelli I, Ferraro A, Esposito F, Pariani E, Inverso D, Montesano C, Delbue S, Perlman S, Tramontano E, De Francesco R, Summa V, Guidotti LG, Iannacone M. EMBO Mol Med. 2023 May 8;15(5):e17580. doi: 10.15252/emmm.202317580.
Total portal vein replacement with peritoneal interposition graft during Whipple's procedure for extrahepatic cholangiocarcinoma: a technical report. Marino R, Tudisco A, Ratti F, Pedica F, Aldrighetti L. World J Surg Oncol. 2023 Mar 29;21(1):117. doi: 10.1186/s12957-023-02995-x.
Case report: Ponatinib as a bridge to CAR-T cells and subsequent maintenance in a patient with relapsed/refractory Philadelphia-like acute lymphoblastic leukemia. Giglio F, Campodonico E, Lorentino F, Noviello M, Xue E, Greco R, Lazzari L, Bruno A, Lupo Stanghellini MT, Carrabba MG, La Starza R, Casucci M, Bonini C, Chiaretti S, Peccatori J, Foà R, Ciceri F. Front Oncol. 2023 Jan 10;12:1100105. doi: 10.3389/fonc.2022.1100105. eCollection 2022.
Neuropilin-1 and Integrins as Receptors for Chromogranin A-Derived Peptides. Corti A, Anderluzzi G, Curnis F. Pharmaceutics. 2022 Dec; 14(12): 2555. doi: 10.3390/pharmaceutics14122555.
Expression of inducible factors reprograms CAR-T cells for enhanced function and safety.Smole A, Benton A, Poussin MA, Eiva MA, Mezzanotte C, Camisa B, Greco B, Sharma P, Minutolo NG, Gray F, Bear AS, Baroja ML, Cummins C, Xu C, Sanvito F, Goldgewicht AL, Blanchard T, Rodriguez-Garcia A, Klichinsky M, Bonini C, June CH, Posey AD Jr, Linette GP, Carreno BM, Casucci M, Powell DJ Jr. Cancer Cell. 2022 Dec 12;40(12):1470-1487.e7.
Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated Tregs as a potential therapeutic target. Alvisi G, Termanini A, Soldani C, Portale F, Carriero R, Pilipow K, Costa G, Polidoro M, Franceschini B, Malenica I, Puccio S, Lise V, Galletti G, Zanon V, Colombo FS, De Simone G, Tufano M, Aghemo A, Di Tommaso L, Peano C, Cibella J, Iannacone M, Roychoudhuri R, Manzo T, Donadon M, Torzilli G, Kunderfranco P, Di Mitri D, Lugli E, Lleo A. J Hepatol. 2022 Nov;77(5):1359-1372.
Continuous sensing of IFNα by hepatic endothelial cells shapes a vascular antimetastatic barrier. Tran NL*, Ferreira LM*, Alvarez-Moya B*, Buttiglione V, Ferrini B, Zordan P, Monestiroli A, Fagioli C, Bezzecchi E, Scotti GM, Esposito A, Leone R, Gnasso C, Brendolan A, Guidotti LG, Sitia G. Elife. 2022 Oct 25;11:e80690. doi: 10.7554/eLife.80690. (* equal contribution)
Cellular and transcriptional dynamics of human neutrophils at steady state and upon stress. Montaldo E, Lusito E, Bianchessi V, Caronni N, Scala S, Basso-Ricci L, Cantaffa C, Masserdotti A, Barilaro M, Barresi S, Genua M, Vittoria FM, Barbiera G, Lazarevic D, Messina C, Xue E, Marktel S, Tresoldi C, Milani R, Ronchi P, Gattillo S, Santoleri L, Di Micco R, Ditadi A, Belfiori G, Aleotti F, Naldini MM, Gentner B, Gardiman E, Tamassia N, Cassatella MA, Hidalgo A, Kwok I, Ng LG, Crippa S, Falconi M, Pettinella F, Scapini P, Naldini L, Ciceri F, Aiuti A, Ostuni R. Nat Immunol. 2022 Oct;23(10):1470-1483. doi: 10.1038/s41590-022-01311-1.
A Comprehensive Characterization of Stemness in Cell Lines and Primary Cells of Pancreatic Ductal Adenocarcinoma. Ferrara B, Dugnani E, Sordi V, Pasquale V, Pellegrini S, Reni M, Balzano G, Piemonti L. Int J Mol Sci. 2022 Sep 14;23(18):10663. doi: 10.3390/ijms231810663.
TCR-engineered iNKT cells induce robust antitumor response by dual targeting cancer and suppressive myeloid cells. Delfanti G, Cortesi F, Perini A, Antonini G, Azzimonti L, de Lalla C, Garavaglia C, Squadrito ML, Fedeli M, Consonni M, Sesana S, Re F, Shen H, Dellabona P, Casorati G. Sci Immunol. 2022 Aug 12;7(74):eabn6563. doi: 10.1126/sciimmunol.abn6563.
Targeting the Blood-Brain Tumor Barrier with Tumor Necrosis Factor-α. Corti A, Calimeri T, Curnis F, Ferreri AJM.
Pharmaceutics. 2022 Jul 6;14(7):1414. doi: 10.3390/pharmaceutics14071414.
CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome. Arcangeli S, Bove C, Mezzanotte C, Camisa B, Falcone L, Manfredi F, Bezzecchi E, El Khoury R, Norata R, Sanvito F, Ponzoni M, Greco B, Moresco MA, Carrabba MG, Ciceri F, Bonini C, Bondanza A, Casucci M. J Clin Invest. 2022 Jun 15;132(12):e150807.
Workflow for high-dimensional flow cytometry analysis of T cells from tumor metastases. Faccani C, Rotta G, Clemente F, Fedeli M, Abbati D, Manfredi F, Potenza A, Anselmo A, Pedica F, Fiorentini G, Villa C, Protti MP, Doglioni C, Aldrighetti L, Bonini C, Casorati G, Dellabona P, de Lalla C. Life Science Alliance. Jun 2022, 5 (10) e202101316. doi: 10.26508/lsa.202101316.
Drugging inflammation: Easier NSAID than done. Caronni N, Ostuni R. Immunity. 2022 Jun 14;55(6):973-975. doi: 10.1016/j.immuni.2022.05.009.
Heterogeneity of tissue resident memory T cells. Konjar Š, Ficht X, Iannacone M, Veldhoen M. Immunol Lett. 2022 May;245:1-7.
Immunological insights in the treatment of chronic hepatitis B. Iannacone M, Andreata F, Guidotti LG. Curr Opin Immunol. 2022 May 16;77:102207. doi: 10.1016/j.coi.2022.102207.
Food-Grade Titanium Dioxide Induces Toxicity in the Nematode Caenorhabditis elegans and Acute Hepatic and Pulmonary Responses in Mice. Sitia G, Fiordaliso F, Violatto MB, Alarcon JF, Talamini L, Corbelli A, Ferreira LM, Tran NL, Chakraborty I, Salmona M, Parak WJ, Diomede L, Bigini P. Nanomaterials (Basel). 2022 May 13;12(10):1669. doi: 10.3390/nano12101669.
Adoptive Immunotherapy With Engineered iNKT Cells to Target Cancer Cells and the Suppressive Microenvironment. Delfanti G, Dellabona P, Casorati G, Fedeli M. Front Med (Lausanne). 2022 May 9;9:897750. doi: 10.3389/fmed.2022.897750.
Heterogeneity in antiviral B cell responses: Lessons from the movies. Kuka M, Iannacone M. Immunol Rev. 2022 Mar;306(1):224-233.
Group 1 ILCs regulate T cell-mediated liver immunopathology by controlling local IL-2 availability. Fumagalli V, Venzin V, Di Lucia P, Moalli F, Ficht X, Ambrosi G, Giustini L, Andreata F, Grillo M, Magini D, Ravà M, Friedrich C, Fontenot JD, Bousso P, Gilmore SA, Khan S, Baca M, Vivier E, Gasteiger G, Kuka M, Guidotti LG†, Iannacone M†. Sci Immunol. 2022 Feb 25;7(68):eabi6112. doi: 10.1126/sciimmunol.abi6112. (†Co-Last Authors)
Arenaviral infection causes bleeding in mice due to reduced serotonin release from platelets. Aiolfi R*, Sitia G*, Iannacone M, Brunetta I, Guidotti LG, Ruggeri ZM. Science Signaling 2022 Feb 22;15(722):eabb0384. doi: 10.1126/scisignal.abb0384. (*equal contribution)
CRISPR-based gene disruption and integration of high-avidity, WT1-specific T cell receptors improve anti-tumor T cell function. Ruggiero E, Carnevale E, Prodeus A, Magnani ZI, Camisa B, Merelli I, Politano C, Stasi L, Potenza A, Cianciotti BC, Manfredi F, Di Bono M, Vago L, Tassara M, Mastaglio S, Ponzoni M, Francesca Sanvito, Liu D, Balwani I, Galli R, Genua M, Ostuni R, Doglio M, O'Connell D, Dutta I, Yazinski SA, McKee M, Arredouani MS, Schultes B, Ciceri F, Bonini C. Sci Transl Med. 2022 Feb 9;14(631):eabg8027. doi: 10.1126/scitranslmed.abg8027.
Adoptive T cell therapy of solid tumors: time to team up with immunogenic chemo/radiotherapy. Pocaterra A, Catucci M, and Mondino A. Curr Opin Immunol. 2022 Feb;74:53-59. doi: 10.1016/j.coi.2021.10.004.
Interrupting the nitrosative stress fuels tumor-specific cytotoxic T lymphocytes in pancreatic cancer. De Sanctis F, Lamolinara A, Boschi F, Musiu C, Caligola S, Trovato R, Fiore A, Frusteri C, Anselmi C, Poffe O, Cestari T, Canè S, Sartoris S, Giugno R, Del Rosario G, Zappacosta B, Del Pizzo F, Fassan M, Dugnani E, Piemonti L, Bottani E, Decimo I, Paiella S, Salvia R, Lawlor RT, Corbo V, Park Y, Tuveson DA, Bassi C, Scarpa A, Iezzi M, Ugel S, Bronte V. J Immunother Cancer. 2022 Jan;10(1):e003549. doi: 10.1136/jitc-2021-003549.
Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy 1 against solid malignancies. Greco B, Malacarne V, De Girardi F, Scotti GM, Manfredi F, Angelino E, Sirini C, Camisa B, Falcone L, Moresco MA, Paolella K, Di Bono M, Norata R, Sanvito F, Arcangeli S, Doglioni C, Ciceri F, Bonini C, Graziani A, Bondanza A, Casucci M. Sci Transl Med. 2022 Jan 19;14(628):eabg3072.. doi: 10.1126/scitranslmed.abg3072.
Administration of aerosolized SARS-CoV-2 to K18-hACE2 mice uncouples respiratory infection from fatal neuroinvasion. Fumagalli V, Ravà M, Marotta D, Di Lucia P, Laura C, Sala E, Grillo M, Bono E, Giustini L, Perucchini C, Mainetti M, Sessa A, Garcia-Manteiga JM, Donnici L, Manganaro L, Delbue S, Broccoli V, De Francesco R, D'Adamo P, Kuka M, Guidotti LG†, Iannacone M†. Sci Immunol. 2021 Nov 23:eabl9929. doi: 10.1126/sciimmunol.abl9929. (†Co-Last Authors)
Chromatin Velocity reveals epigenetic dynamics by single-cell profiling of heterochromatin and euchromatin. Tedesco M, Giannese F, Lazarević D, Giansanti V, Rosano D, Monzani S, Catalano I, Grassi E, Zanella ER, Botrugno OA, Morelli L, Panina Bordignon P, Caravagna G, Bertotti A, Martino G, Aldrighetti L, Pasqualato S, Trusolino L, Cittaro D, Tonon G. Nat Biotechnol. 2021 Oct 11.doi: 10.1038/s41587-021-01031-1.
Posttransplantation Cyclophosphamide- and Sirolimus-Based Graft-Versus-Host-Disease Prophylaxis in Allogeneic Stem Cell Transplant. Greco R, Lorentino F, Albanese S, Stanghellini MTL, Giglio F, Piemontese S, Clerici D, Lazzari L, Marcatti M, Mastaglio S, Xue E, Farina F, Pavesi F, Assanelli A, Carrabba MG, Marktel S, Vago L, Bonini C, Corti C, Bernardi M, Ciceri F, Peccatori J. Transplant Cell Ther 2021 09; 27: 776.e1-776.e13. doi: 10.1016/j.jtct.2021.05.023.
miR-21 sustains CD28 signalling and low-affinity T-cell responses at the expense of self-tolerance. Fedeli M, Kuka M, Finardi A, Albano F, Viganò V, Iannacone M, Furlan R, Dellabona P, Casorati G. Clin Transl Immunology. 2021 Sep 21;10(9):e1321. doi: 10.1002/cti2.1321.
COVID-eVax, an electroporated DNA vaccine candidate encoding the SARS-CoV-2 RBD, elicits protective responses in animal models. Conforti A, Marra E, Palombo F, Roscilli G, Ravà M, Fumagalli V, Muzi A, Maffei M, Luberto L, Lione L, Salvatori E, Compagnone M, Pinto E, Pavoni E, Bucci F, Vitagliano G, Stoppoloni D, Pacello ML, Cappelletti M, Ferrara FF, D'Acunto E, Chiarini V, Arriga R, Nyska A, Di Lucia P, Marotta D, Bono E, Giustini L, Sala E, Perucchini C, Paterson J, Ryan KA, Challis AR, Matusali G, Colavita F, Caselli G, Criscuolo E, Clementi N, Mancini N, Groß R, Seidel A, Wettstein L, Münch J, Donnici L, Conti M, De Francesco R, Kuka M, Ciliberto G, Castilletti C, Capobianchi MR, Ippolito G, Guidotti LG, Rovati L, Iannacone M, Aurisicchio L.
Mol Ther. 2021 Sep 20:S1525-0016(21)00466-4. doi: 10.1016/j.ymthe.2021.09.011.
Identification of a Kupffer cell subset capable of reverting the T cell dysfunction induced by hepatocellular priming. De Simone G, Andreata F, Bleriot C, Fumagalli V, Laura C, Garcia-Manteiga JM, Di Lucia P, Gilotto S, Ficht X, De Ponti FF, Bono EB, Giustini L, Ambrosi G, Mainetti M, Zordan P, Bénéchet AP, Ravà M, Chakarov S, Moalli F, Bajenoff M, Guidotti LG, Ginhoux F, Iannacone M. Immunity. 2021 Sep 14;54(9):2089-2100.e8. doi: 10.1016/j.immuni.2021.05.005.
Isolation of mouse Kupffer cells for phenotypic and functional studies. Andreata F, Blériot C, Di Lucia P, De Simone G, Fumagalli V, Ficht X, Beccaria CG, Kuka M, Ginhoux F, Iannacone M. STAR Protoc. 2021 Sep 14;2(4):100831. doi: 10.1016/j.xpro.2021.100831.
The Extracellular Matrix in Pancreatic Cancer: Description of a Complex Network and Promising Therapeutic Options. Ferrara B, Pignatelli C, Cossutta M, Citro A, Courtyì J, Piemonti L. Cancers (Basel). 2021 Sep 3;13(17):4442. doi: 10.3390/cancers13174442.
CD4+ T cells sustain aggressive chronic lymphocytic leukemia in Eμ-TCL1 mice through a CD40L-independent mechanism. Grioni M, Brevi A, Cattaneo E, Rovida A, Bordini J, Bertilaccio MTS, Ponzoni M, Casorati G, Dellabona P, Ghia P, Bellone M,Calcinotto A. Blood Adv 2021 07; 5: 2817-2828. doi: 10.1182/bloodadvances.2020003795.
Laparoscopic Surgery for Intrahepatic Cholangiocarcinoma: A Focus on Oncological Outcomes. Ratti F, Casadei-Gardini A, Cipriani F, Fiorentini G, Pedica F, Burgio V, Cascinu S, Aldrighetti L. J Clin Med. 2021 Jun 26;10(13):2828. doi: 10.3390/jcm10132828.
A PGE2-MEF2A axis enables context-dependent control of inflammatory gene expression. Cilenti F*, Barbiera G*, Caronni N, Iodice D, Montaldo E, Barresi E, Lusito E, Cuzzola V, Vittoria FM, Mezzanzanica L, Miotto P, Di Lucia P, Lazarevic D, Cirillo DM, Iannacone M, Genua M^, Ostuni R^. IMMUNITY. 2021 June 14. doi:10.1016/j.immuni.2021.05.01. (*= equal contribution ^=equal contribution)
Flow cytometry data mining by cytoChain identifies determinants of exhaustion and stemness in TCR-engineered T cells. Manfredi F, Abbati D, Cianciotti BC, Stasi L, Potenza A, Ruggiero E, Magnani Z, Carnevale E, Doglio M, Noviello M, Tassi E, Balestrieri C, Buonanno S, Clemente F, De Lalla C, Protti MP, Mondino A, Casorati G, Dellabona P, Bonini C. Eur J Immunol. 2021 Jun 3. doi: 10.1002/eji.202049103.
Nanogold functionalized with lipoamide-isoDGR: a simple, robust and versatile nanosystem for avb3-integrin targeting Sacchi A, Gasparri AM, Monieri M, Anderluzzi G , Colombo B , Gori A, Corti A* and Curnis F*. Front Chem. 2021 May 28;9:690357. doi: 10.3389/fchem.2021.690357. (* Co-Corresponding Author)
Organosilica Cages Target Hepatic Sinusoidal Endothelial Cells Avoiding Macrophage Filtering. Talamini L, Picchetti P, Ferreira LM, Sitia G, Russo L, Violatto MB, Travaglini L, Fernandez Alarcon J, Righelli L, Bigini P and De Cola L. ACS Nano. 2021 May 19. doi:10.1021/acsnano.1c00316.
Immunobiology and pathogenesis of hepatitis B virus infection. Iannacone M, Guidotti LG. Nat Rev Immunol. 2021 May 17. doi: 10.1038/s41577-021-00549-4.
Enhancement of doxorubicin anti-cancer activity by vascular targeting using IsoDGR/cytokine-coated nanogold. Corti A*, Sacchi A, Gasparri AM, Monieri M , Anderluzzi G, Colombo B, Gori A, Mondino A and Curnis F*. J Nanobiotechnology. 2021 May 5;19(1):128. doi:10.1186/s12951-021-00871-y. (* Co-Corresponding Author)
Exploiting CD1-restricted T cells for clinical benefit. Exley MA, Dellabona P, Casorati G.MOL IMMUNOL 2021 Feb; 132: 126-131. doi: 10.1016/j.molimm.2020.12.015.
Evolution of surgical treatment of Colorectal Liver Metastases 2in the real world: single center experience in 1212 cases. Ratti F, Cipriani F, Fiorentini G, Burgio V, Ronzoni M, Della Corte A, Cascinu S, De Cobelli F, and Aldrighetti L. CANCERS 2021 Mar 9;13(5):1178. doi: 10.3390/cancers13051178.
Determinants, mechanisms, and outcomes of myeloid cell diversity in cancer. Caronni N, Montaldo E, Mezzanzanica L, Cilenti F, Genua M, Ostuni R. IMMUNOL REV 2021 Feb 9. doi: 10.1111/imr.12944.
Advances in Bio-Based Polymers for Colorectal CancerTreatment: Hydrogels and Nanoplatforms. Maspes A, Pizzetti F, Rossetti A, Makvandi P, Sitia G, Rossi F. Gels. 2021 Jan 11;7(1):6. doi: 10.3390/gels7010006.
Circulating chromogranin A is cleaved into vasoregulatory fragments in patients with pancreatic ductal adenocarcinoma. Reni M, Andreasi V , Gasparri AM , Dugnani E , Colombo B, Macchini M, Bianco M, Dallatomasina A, Citro A, Assi E, Protti MP, Esposito A, Falconi M, Curnis F, Piemonti L, and Corti A. Front Oncol. 2020 Dec 23;10:613582. doi: 10.3389/fonc.2020.613582.
Serum HBsAg clearance has minimal impact on CD8+ T cell responses in mouse models of HBV infection. Fumagalli V, Di Lucia P, Venzin V, Bono EB, Jordan R, Frey CR, Delaney W, Chisari FV, Guidotti LG†, Iannacone M†. J Exp Med. 2020 Nov 2;217(11):e20200298. doi: 10.1084/jem.20200298. (†Co-Last Authors)
NGR-TNF Engineering with an N-Terminal Serine Reduces Degradation and Post-Translational Modifications and Improves Its Tumor-Targeting Activity. Corti A, Gasparri AM, Sacchi A, Colombo B, Monieri M, Rrapaj E, Ferreri AJM, Curnis F. Mol Pharm. 2020 Oct 5;17(10):3813-3824. doi: 10.1021/acs.molpharmaceut.0c00579.
Thymic stromal lymphopoietin and Cancer: Th2-dependent and -independent mechanisms. Protti MP, De Monte L. Front Immunol. 2020 Sep 16;11:2088. doi: 10.3389/fimmu.2020.02088.
Immune surveillance of the liver by T cells. Ficht X, Iannacone M. Sci Immunol. 2020 Sep 4;5(51):eaba2351. doi: 10.1126/sciimmunol.aba2351.
TCR redirected T cells for cancer treatment: Acheivements, hurdles and goals. Manfredi F, Cianciotti BC, Potenza A, Tassi E, Noviello E, Biondi A, Ciceri F, Bonini C*, Ruggiero E.*Front Immunol. 2020 Sep 3;11:1689. doi: 10.3389/fimmu.2020.01689. (*= equal contribution)
Opportunities and challenges associated with the evaluation of chimeric antigen receptor T cells in real-life. McGrath E, Chabannon C, Terwel S, Bonini C, Kuball J. Current Opinion in Oncology: Sep 2020 - Volume 32 - Issue 5 - p 427-433 doi: 10.1097/CCO.0000000000000665.
Overcoming key challenges in engineered T cell cancer immunotherapy. Arcangeli S*, Mestermann K*, Weber J*, Bonini C^, Casucci M^, Michael M.^ Current Opinion in Oncology. 2020 Sep;32(5):398-407. doi: 10.1097/CCO.0000000000000664. (*= equal contribution ^=equal contribution).
Spatiotemporal regulation of type I interferon expression determines the antiviral polarization of CD4+ T cells. De Giovanni M, Cutillo V, Giladi A, Sala E, Maganuco CG, Medaglia C, Di Lucia P, Bono E, Cristofani C, Consolo E, Giustini L, Fiore A, Eickhoff S, Kastenmüller W, Amit I, Kuka M, Iannacone M. NAT IMMUNOL 2020 Mar; 21: 321-330. doi: 10.1038/s41590-020-0596-6.
Dual Role of Inflammasome Adaptor ASC in Cancer. Protti, MP and De Monte L. Front Cell Dev Bio 2020 Feb; 8: Article 40. doi: 10.3389/fcell.2020.00040.
A stapled chromogranin A-derived peptide is a potent dual ligand for integrins αvβ6 and αvβ8. Nardelli F, Ghitti M, Quilici G, Gori A, Luo Q, Berardi A, Sacchi A, Monieri M, Bergamaschi G, Bermel W, Chen F, Corti A, Curnis F, Musco G. CHEM COMMUN 2019 Dec; 55: 14777-14780. doi: 10.1039/c9cc08518a.
Chromogranin A and its fragments in cardiovascular, immunometabolic, and cancer regulation Mahata SK, Corti A. ANN NY ACAD SCI 2019 Nov; 1455: 34-58. doi: 10.1111/nyas.14249.
Boosting Interleukin-12 Antitumor Activity and Synergism with Immunotherapy by Targeted Delivery with isoDGR-Tagged Nanogold. Gasparri AM, Sacchi A, Basso V, Cortesi F, Freschi M, Rrapaj E, Bellone M, Casorati G, Dellabona P, Mondino A, Corti A, Curnis F. SMALL 2019 Nov; 15: e1903462. doi: 10.1002/smll.201903462.
Development of adaptive immune effector therapies in solid tumors. Comoli P, Chabannon C, Koehl U, Lanza F, Urbano-Ispizua A, Hudecek M, Ruggeri A, Secondino S, Bonini C, Pedrazzoli P. Ann Oncol. 2019 Nov 1;30(11):1740-1750. doi: 10.1093/annonc/mdz285.
Dynamics and genomic landscape of CD8+ T cells undergoing hepatic priming Bénéchet AP, De Simone G, Di Lucia P, Cilenti F, Barbiera G, Le Bert N, Fumagalli V, Lusito E, Moalli F, Bianchessi V, Andreata F, Zordan P, Bono E, Giustini L, Bonilla WV, Bleriot C, Kunasegaran K, Gonzalez-Aseguinolaza G, Pinschewer DD, Kennedy PTF, Naldini L, Kuka M, Ginhoux F, Cantore A, Bertoletti A, Ostuni R, Guidotti LG, Iannacone M. Nature. 2019 Oct;574(7777):200-205. doi: 10.1038/s41586-019-1620-6.
Total Publications: 122
Total IF: 2345.489
Mean IF: 19.877
Last update: 23/12/2025