San Raffaele Telethon Institute for Gene Therapy
San Raffaele Telethon Institute for Gene Therapy


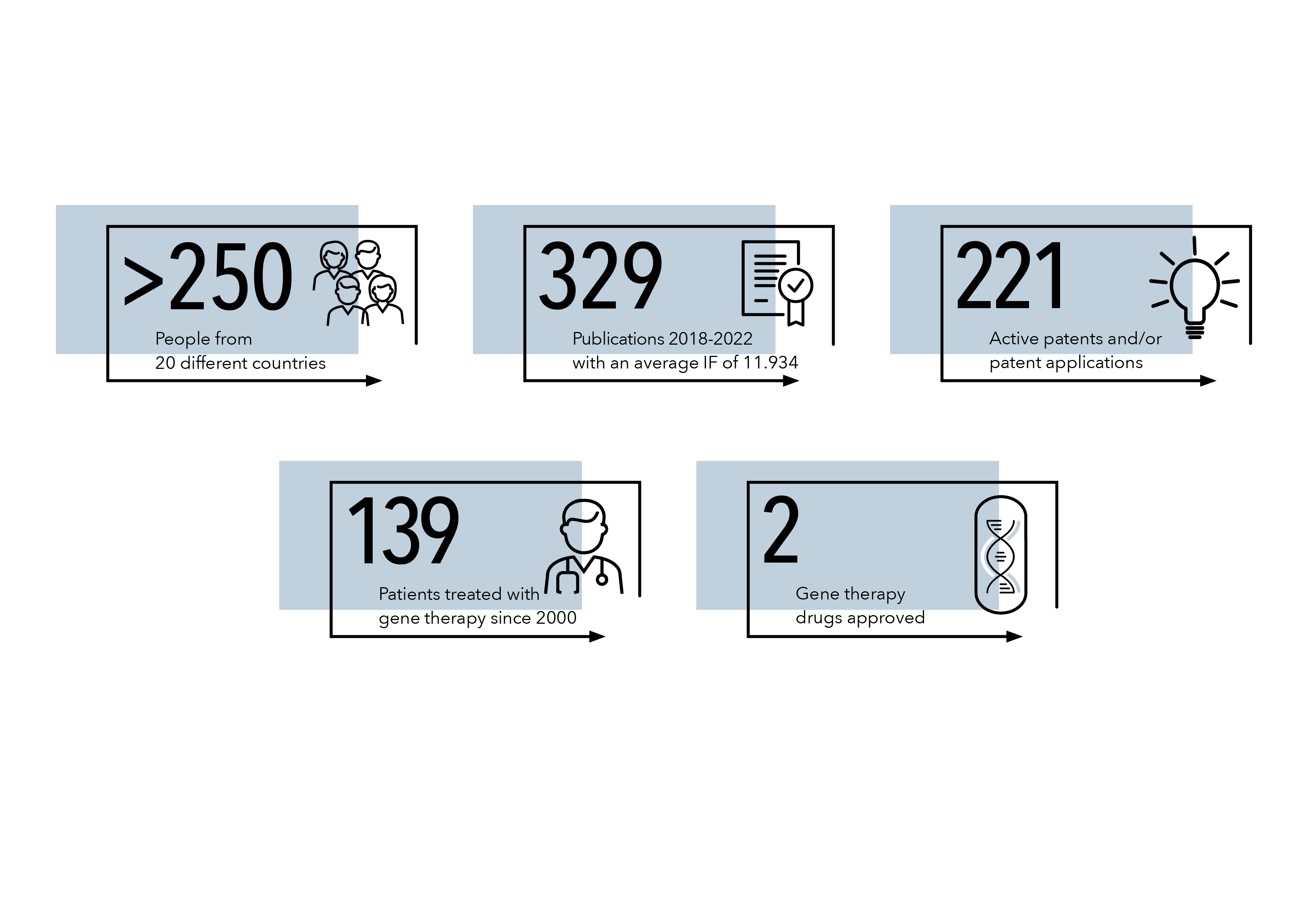
The San Raffaele Telethon Institute for Gene Therapy (SR-Tiget) was created in 1996 as a joint venture between the Fondazione Telethon and Ospedale San Raffaele, with the mission to perform cutting-edge research in gene and cell therapy and to translate its results into therapeutic advances, focusing on genetic diseases.
SR-Tiget represents a multi-disciplinary research environment, which provides a unique blend of scientific expertise in the development of innovative gene and cell therapy strategies, access to relevant preclinical models to evaluate their efficacy and safety, as well as competence in conducting early phase clinical trials. This provides a fertile ground for alliances with industrial partners and launch of start-up companies, which are crucial to secure the skills and resources required to address the regulatory hurdles and manufacturing needs to bring new therapies to registration and make them available to patients.
Download here the brochure | Contact us.
GOALS & ASSETTS
We employ a basic-to-translational, bench-to-bedside R&D approach encompassing the characterization of biological properties and physiopathological processes, the design and optimization of safety and efficacy of novel gene and cell therapy platforms and their development from preclinical models to first-in-human testing.
| GOAL 1: Advance HSC gene therapy to standard-of-care for rare genetic diseases | Discover more |
GOAL 2: Be at the forefront of gene editing development | Discover more |
| GOAL 3: Broaden application of lentiviral vectors to in vivo gene therapy | Discover more | GOAL 4: Devise new cell and gene therapy strategies to modulate immune response | Discover more |
Recognized assets of the Institute include:
- Leadership in lentiviral gene transfer technology
- Pioneering contribution to the genome and epigenome editing field
- State-of-the-art stem cell transplantation center for adult and pediatric patients
- In house facilities and resources supporting translational research and first-in-human phase I/II gene therapy trials
- Strategic alliances with industrial partners and successful launch of startup biotech companies
- Roster of young PIs venturing into relevant new technologies and emerging biological concepts
Alessandro Aiuti
Group leader, Pathogenesis and therapy of primary immunodeficiencies Unit
Alessio Cantore
Group leader, Liver gene therapy Unit
Raffaella Di Micco
Group leader, Senescence in stem cell aging, differentiation and cancer Unit
Andrea Ditadi
Group leader, Human hematopoietic development and disease modeling Unit
Giuliana Ferrari
Group leader, Gene transfer into stem cell Unit
Silvia Gregori
Group leader, Mechanisms of peripheral tolerance Unit
Angela Gritti
Group leader, Gene and neural stem cell therapy for lysosomal storage diseases Unit
Angelo Lombardo
Group leader, Epigenetic regulation and targeted genome editing Unit
Eugenio Montini
Group leader, Safety of gene therapy and insertional mutagenesis Unit
Alessandra Mortellaro
Group leader, Mechanisms of inflammation in health and disease Unit
Luigi Naldini
Group leader, Novel Gene Therapy Strategies Unit
Renato Ostuni
Group leader, Genomics of the innate immune system Unit
Anna Villa
Group leader, Pathogenesis and treatment of immune and bone diseases Unit
Michele Gabriele
SR-Tiget Group Leader, 3D genome dynamics in differentiation and disease
Mario Squadrito
SR-Tiget Group Leader, Vector Engineering and In vivo Tumor Targeting
Gabriele Ciceri
SR-Tiget Group Leader, Stem Cells & Timing of Neural Development in Health and Disease
Daniela Cesana
Group Leader, Tissue Dynamics and Biomarker Signature Discovery
The SR-Tiget portfolio of gene and cell therapies embraces the full spectrum of drug development. The most advanced steps of the Institute development pipeline are undertaken in the context of strategic alliances with industrial partners or through the spin-off of startup biotechnology companies, which are crucial to secure the resources and the multi‐disciplinary expertise required to attain the ultimate goal of delivering the therapies to patients.
Starting from 2004, the Institute has been involved in a number of strategic alliances set up between OSR, Fondazione Telethon and key industry players in the cell and gene therapy field, including:
- Orchard Therapeutics Limited (2018-ongoing)
- Sanofi (2017-2022)
- Editas Medicine (2016-2018)
- Biogen (2014-2017)
- GlaxoSmithKline (2010-2018)
- Sangamo Therapeutics (2004-2015)
More recently, three startup companies stemmed from SR-Tiget research:
- Genenta Science (launched by OSR), focused on the development of cancer immunotherapy. In 2021, the company was listed on Nasdaq, becoming the first italian biotech on the American stock exchange market.
- Epsilen Bio (launched by OSR and Fondazione Telethon), whose mission is to develop therapies based on the epigenetic silencing platform. In 2021, it has been acquired by Chroma Medicine, giving rise to the largest epigenetic editing company in the world.
- Genespire (launched by OSR and Fondazione Telethon), whose portfolio includes the development of ex vivo gene editing therapies and liver-directed in vivo gene therapies based on advanced lentiviral vectors.
TRAINING
SR-Tiget actively promotes the professional growth of scientists at all stages of their career.
Undergraduate students. SR-Tiget laboratories offer internship opportunities to motivated master students from all universities. Trainees benefit from a dynamic scientific environment and have access to activities organized by the host labs and the Institute (e.g. journal clubs, seminars, retreats, etc.).
PhD students. The Institute hosts PhD students mainly enrolled in the International PhD Course in Molecular Medicine at the Vita-Salute San Raffaele University. In particular a dedicated Gene and Cell Therapy curriculum aims to train young scientists in the study of genetic diseases and in the development of new gene and cell therapy strategies.
Postdoctoral Fellows. SR-Tiget is committed to support postdoctoral researchers in their professional development, providing training aimed at broadening both their research and soft skills and preparing them for the next step of their career.